
Vaidyanathan Hariharan
- November 25, 2021
- 0

Test procedure for JIS A-1460 desiccator consists of following steps:
i. Stabilising 2 set of test samples by conditioning to a consistent mass.
ii. Making & standardising formaldehyde stock solution.
iii. Using standardised formaldehyde stock dilutions for making calibration curve.
iv. Spectrophotometer reading and making the calibration curve.
v. Standardising Sodium Thiosulphate solution.
vi. Making fresh reagents for reaction procedures.
vii. 24 hour desiccator procedure for – blank, sample set 1, sample set 2.
viii. Spectrophotometer readings and calculations.
i. Stabilising of test samples by conditioning
Test pieces are cut to size precisely at 150 (±1)mm X 50 (±1)mm so that the total surface area (top, bottom, all four sides) of test pieces is as close to 1800cm2 as possible. The test pieces are then conditioned at 20±2oC & 65±5% RH with a gap of 25mm between test pieces, in a chamber, until the pieces attain constant mass. Constant mass is achieved when weight of 2 successive weight measurement has <0.1% difference, OR, conditioning is assumed to be completed after a period of 7 days from start of conditioning procedure.
ii. Making & standardising formaldehyde stock solution
Formaldehyde standard solution is prepared in a volumetric flask so that 3mg of formaldehyde is contained in 1000mL of the water, to the marked line. Precisely 1mL of formaldehyde (37% – lab grade) is made up to 1000mL in a volumetric flask using distilled water.
Standardisation (To measure exact content of formaldehyde in the above prepared standard solution):
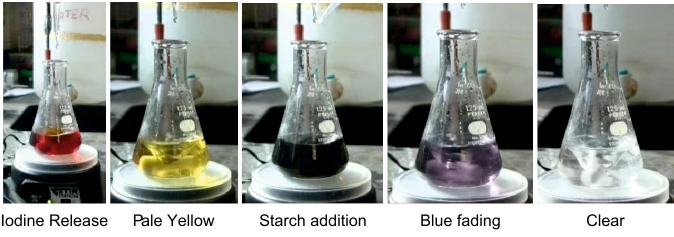
20mL of the standard solution is taken into a clean & dry 100mL conical flask with glass stopper (Erlenmeyer Flask). 25mL of 0.05M Iodine solution is added, followed by 10mL of 1M sodium hydroxide solution with slow stirring. This is left in the dark for exactly 15 minutes. After this time, it is taken out and 15mL of 1M sulphuric acid is added & immediately titrated against 0.1M sodium thiosulphate solution taken in the burette. Titrate until colour of solution becomes pale yellow. Add 1mL fresh, room temperature starch solution, and continue titrating until the blue colour disappears completely (first disappearance to clear solution). Use the same procedure with 20mL blank (distilled water) also.
C = 1.5 x (V0 – V) x f x 1000/20
C = concentration of HCHO in the prepared standard solution (mg/L)
1.5 = quantity of HCHO corresponding to 1mL of 0.1M sod thiosulphate solution (mg)
V0 = titration value of 0.1M sod thiosulphate solution in blank test (mL)
V = titration value of 0.1M sod thiosulphate solution in the prepared HCHO standard solution (mL)
f = factor of 0.1M sod thiosulphate solution (standardisation value / 0.1)
i. Using standardised formaldehyde stock dilutions for making calibration curve
0, 5, 10, 20, 50, & 100mL of the standard HCHO stock solutions are precisely pipette into 100mL volumetric flasks & made up to the mark with deionised water. These 6 set of dilutions are used to make the calibration curve & obtain the slope of calibration curve.
NOTE: If the concentration of formaldehyde exceeds the range of calibration curve (0 to 3mg), the solution has to be diluted to obtain the precise concentration in this range.
ii. Spectrophotometer reading and making the calibration curve
25mL of each dilution as above (iii) is taken into a 100mL Erlenmeyer flask. 25mL of acetylacetone-ammonium acetate solution is added and mixed with stopper on. The flask is heated with light mixing in a water-bath at 65±2oC for 10 minutes, and then allowed to stand under shaded condition until it reaches room temperature. This solution is taken into an absorption cell & absorbance is measured at 412 nm wavelength using spectrophotometer. Sometimes, maximum absorbance value is observed at 410 nm. In such a case, all values are taken at this wavelength.
Graph is plotted correlating quantity of formaldehyde (0 to 3mg) with absorbance. Slope is calculated either by graphical method or by calculation of slope of curve.
(Next Issue: Test procedure & Calculations…contd)
































































